|
Entomotropica
Vol. 18(2): 79-82. Agosto 2003
|
ISSN 1317-5262
|
Composition of the defensive secretion of the Neotropical millipede Rhinocricus padbergi Verhoeff 1938 (Diplopoda: Spirobolida: Rhinocricidae)
Alberto Arab1, Giuliano G. Zacarin1, Carmen S. Fontanetti1, Maria Izabel Camargo-Mathias1, Márcio G. dos Santos2, Aivlé C. Cabrera3
1Departamento de Biologia, Universidade Estadual Paulista (UNESP), 13506-900, Rio Claro, SP, Brazil, e-mail: [email protected] 2Departamento de Química, Universidade Federal de São Carlos, 13565-905, São Carlos, SP, Brazil.
3Laboratorio de Comportamiento. Universidad Simón Bolívar, 89000, Caracas, 1080-A, Venezuela.
Recibido: 08-xi-2002
Aceptado: 09-iv-2003
Correcciones devueltas por el autor: 23-v-2003
Abstract
Arab A, Zacarin GG, Fontanetti CS, Camargo-Mathias MI, Dos Santos MG, Cabrera AC. 2003. Composition of the defensive secretion of the Neotropical millipede Rhinocricus padbergi Verhoeff 1938 (Diplopoda: Spirobolida: Rhinocricidae). Entomotropica 18(2):79-82.
The components of extracts of the repugnatorial glands were identified in the millipede R. padbergi. Hexane extracts of 50 glands were prepared and analyzed by gas cromatography-mass spectrometry (GC-MS). We identified the two main volatile compounds: methyl-1,4-benzoquinone and 3,3a,4,5-tetrahydro-1H-pyrrolo-[2,3-b] pyridine-2,6-dione. The significance of these compounds in the secretion of the repugnatorial glands is discussed.
Additional key words: Alkaloids, benzoquinones, chemical defense, repugnatorial glands.
Resumen
Arab A, Zacarin GG, Fontanetti CS, Camargo-Mathias MI, Dos Santos MG, Cabrera AC. 2003. Composición de las secreciones de defensa del diplópodo Neotropical Rhinocricus padbergi Verhoeff 1938 (Diplopoda: Spirobolida: Rhinocricidae). Entomotropica 18(2):79-82.
En este trabajo se determinó la composición de los extractos de las glándulas de defensa del diplópodo R. padbergi. Se prepararon extractos de 50 glándulas y se analizaron por GC-MS. Fueron identificados los dos componentes volátiles principales de la secreción glandular: metil-1,4-benzoquinona y 1H-pirrolo-[2,3-b] piridina-2,6-dione 3,3a,4,5-tetrahidro. La relevancia de estos compuestos en la secreción de defensa de un diplópodo es discutida..
Palabras clave adicionales: Alcaloides, benzoquinonas, defensa química, glándulas repugnatorias.
Introduction
Most millipedes (Diplopoda) are slow moving animals and, therefore, they are unable to escape in order to avoid attack. Many species respond to disturbance by coiling up and ejecting a noxious fluid from special glands (Meinwald et al. 1966). Unlike their myriapod relatives, repugnatorial glands are present in all millipedes except for the Penicillata and the Sphaerotheriida. For spirobolid millipedes, there is usually a single pair of glands per segment and the orifice of each gland lies at the dorsum of the posterior lateral region of each segment (Woodring and Blum 1965).
The secretion of the repugnatorial glands of millipedes may have different composition depending on the taxonomic group. Alkaloids are secreted by the Glomerida; phenols by the Callipodida and the Chordeumoidea; benzoquinones by the species of the orders Julida, Spirobolida, and Spirostreptida; hydrogen cyanide, cyanogenic compounds and nitroalkenes by the Polydesmida; and terpenoids by the Colobognatha (Polyzoniidae) (Eisner et al. 1996; Kuwahara et al. 2002). Most of the compounds act as topical irritants to man's skin and other large animals. If ingested, these compounds can be highly toxic and carcinogenic (Eisner et al. 1998). Children and domestic animals are the most affected and may suffer injury to the face or mouth if they attempt to bite or chew a millipede. Eye and skin lesions are common during the manipulation of certain species. There is a severe burning sensation and, in the case of the eyes, intense lacrimation and ulceration of the cornea (Radford 1975).
Rhinocricus padbergi (Spirobolida: Rhinocricidae) is a medium sized millipede that inhabits southeastern Brazil. During the rainy season, these millipedes invade urban areas and can cause nuisance and/or injuries to people and domestic animals if manipulated. For these reasons, this work focuses on the chemical analysis of the compounds secreted by the repugnatorial glands of this species in order to obtain a better understanding of the chemical defense mechanisms of this class of arthropods.
Material and methods
Specimens of R. padbergi were collected from leaf litter at open areas around the campus of the Universidade Estadual Paulista in Rio Claro, São Paulo State (lat 22o 23' S, long 47o 32' W), Brazil. They were cold anaesthetized and dissected in n-hexane. The repugnatorial glands (50) from ten millipedes were extracted and immersed in 1 ml of n-hexane. The chemical analyses were made at the Universidade Federal de São Carlos at the Laboratório de Síntese de Produtos Naturais. The extracts were analyzed by gas chromatography (GC) in a Shimadzu 17-A chromatograph equipped with a DB-5 column (30 m x 0.25 mm i.d., 0.25 µm film thickness J and W Scientific). The temperature program used was 70 oC, 7 oC/min to 250 oC, 5 oC/min to 280 oC held for 10 min. The injection was splitless and its temperature was 250 °C. The interface temperature was 280 °C. GC-MS analysis were performed on a Shimadzu QP5000 spectrometer using helium as carrier gas and a DB-5 column. Injections of 1-2 ml of each splitless gland extract were made at 250 oC using the same temperature program described above. The mass spectra were obtained by electron impact ionization at 70 eV.
Results
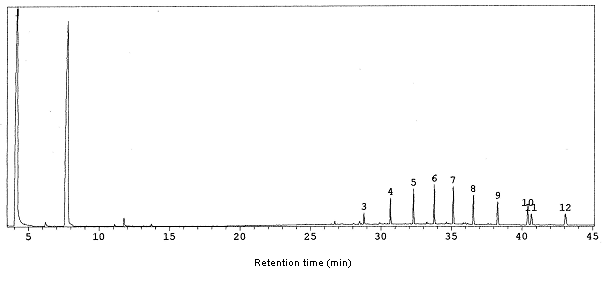
Analyses by GC and GC-MS methods revealed the number of constituents of the extract and allowed the recognition of all the secretion components. The two most volatile components (A and B) of the gland extract had molecular ions at m/z = 122 and 152, respectively. Peak A was at tR 3.8 min and peak B was at tR 7.4 min on GC analysis (Figure 1). They comprised 40% (peak A) and 39% (peak B) of the chromatogram peak-area (Figure 1). These compounds were identified as 2-methyl-1,4-benzoquinone and 3,3a,4,5-tetrahydro-1H-pyrrolo-[2,3-b] pyridine-2,6-dione (Figure 2) by the comparison of the mass spectra and retention times of these compounds. The peaks relative to the compounds 3, 4, 5, 6, 7, 8, 9, 10, 11 and 12 (Figure 1) were identified as a homologous series of linear aliphatic hydrocarbons, beginning with heneicosane [CH3(CH2)19CH 3] and ending in nonacosane [CH3(CH2)27CH 3]. The structural attributions of these compounds were based on their mass spectrum. Co-injection of the gland extracts with hydrocarbons standards [triacontane (C30H62), pentacosane (C25H52), and heneicosane (C21H44)] confirmed the presence of these compounds.
Discussion
Rhinocricus padbergi possess a pair of repugnatorial glands on each diplosegment. Only the first five segments and the two last are devoid of glands. Each gland opens in small pores that run along the flanks of the body. When disturbed, this millipede coils up into a tight spiral, keeping the head in the center. If further disturbed, the millipede discharges a yellow secretion that appears as small droplets over the millipede's body. Our results showed that this secretion is constituted mainly by two compounds: 2-methyl-1,4-benzoquinone and 3,3a,4,5-tetrahydro-1H-pyrrolo-[2,3-b] pyridine-2,6-dione.
Benzoquinones are active components of the defensive secretions of Julida, Spirobolida, and Spirostreptida species (Wodring and Blum 1965; Meinwald et al. 1966). In some species, a combination of various types of these compounds, together with their precursors, can be detected in the same secretion of the repugnatorial glands (Williams et al. 1997; Deml and Huth 2000), which sometimes may show a characteristic pattern (De Bernardi et al. 1982). The compound 2-methyl-1,4-benzoquinone found in R. padbergi has also been found in other species, in which it constitutes the main component of the defensive secretion (Williams et al. 1997; Deml and Huth 2000). This compound, like other benzoquinones, is highly toxic and persistent (Valderrama et al. 2000). Benzoquinones act primarily as a mechanism of defense since they are strongly irritating and repellent to some insects and mammals, which are known as potential predators of millipedes (Santori 1998; Eisner et al. 1998; Valderrama et al. 2000). On the other hand, some studies have shown that benzoquinones are also toxic to a great variety of pathogens and parasites (Williams et al. 1997).
Alkaloids have only been reported in the defensive secretions of Glomerida and Polyzoniidae species (Meinwald et al. 1966; Wood et al. 2000). In general, these compounds are noxious to a great variety of organisms. Due to their complex molecular structure, the biosynthetic pathway of alkaloids is difficult to discern. Although some insects sequester and metabolize plant allelochemicals (mainly alkaloids) to be used in their defense against potential predators (Pasteels et al. 2003), there are no reports concerning synthesis and/or uptake mechanisms of these compounds in millipedes. On the other hand, R. padbergi feed on litter and decayed wood and there is no evidence that this millipede uses plant alkaloids as some herbivorous insects do. To our knowledge, this is the first report of the alkaloid 3,3a,4,5-tetrahydro-1H-pyrrolo-[2,3-b] pyridine-2,6-dione in the defensive secretion of a spirobolid millipede.
|
|
|
Figure 1. Gas chromatogram of the repugnatorial gland extract of the millipede Rhinocricus padbergi, showing 2-methyl-1,4-benzoquinone and 3,3a,4,5-tetrahydro-1H-pyrrolo-[2,3-b] pyridine-2,6-dione (compounds A and B, respectively). Peaks identified with numbers are aliphatic hydrocarbons.
|
|

|
|
Figure 2. Structures of the two most volatile secretory components, 2-methyl-1,4-benzoquinone and 3,3a,4,5-tetrahydro-1H-pyrrolo-[2,3-b] pyridine-2,6-dione (compounds 1 and 2 respectively).
|
The morphology of the repugnatorial glands of R. padbergi consists of a spherical sac bordered by secretory cells lined with cuticle, thus suggesting an integumental origin (Hopkin and Read 1992). Consequently, the presence of aliphatic hydrocarbons in the chemical analyses of the gland secretion may be due to the extraction of some of the cuticular hydrocarbons of these glands. The repugnatorial gland cells of R. padbergi are rich of organelles; the distal portion of these cells showed a high number of secretory granules that are liberated into the spherical sac (Demange 1993). Nevertheless, it remains unknown whether or not these cells are capable of synthesizing their own alkaloids.
In conclusion, the chemical analyses of the repugnatorial gland extracts of R. padbergi showed two main compounds: a benzoquinone and an alkaloid. Benzoquinones are already found in the defensive secretion of many millipedes. However, this is the first report regarding the presence of alkaloids in the defensive secretions of a spirobolid millipede. We consider that further studies are necessary in order to evaluate the biological function of the defensive secretion of R. padbergi and also to discern the biosynthetic pathway of the alkaloid 3,3a,4,5-tetrahydro-1H-pyrrolo-[2,3-b] pyridine-2,6-dione.
Acknowledgments
The authors wish to thank Rogilene Prado for technical assistance and Johana Rincones for reviewing grammar and syntax in the English text.
References
De Bernardi M, Mellerio G, Vidari G, Vita-Finzi P. 1982. Quinones in the defensive secretions of African millipede. Naturwissenschaften 69:601-602.
Demange JM. 1993. Les armes chimiques des mille-pattes. Millepattia 2: 29-61.
Deml R, Huth A. 2000. Benzoquinones and hydroquinones in defensive secretions of tropical millipedes. Naturwissenschaften 87:80-82.
Eisner T, Eisner M, Deyrup M. 1996. Millipede defense: use of detachable bristles to entangle ants. Proc Natl Acad Sci USA 93:10848-10851.
Eisner T, Eisner M, Attygalle AB, Deyrup M, Meinwald J. 1998. Rendering the inedible edible: circumvention of a millipede's chemical defense by a predaceous beetle larva (Phengodidae). Proc Natl Acad Sci USA 95:1108-1113.
Hopkin S, Read H. 1992. The biology of millipedes. New York: Oxford University Press. 233 p.
Kuwahara Y, Ômura H, Tanabe T. 2002. 2-Nitroethenylbenzenes as natural products in millipede defense secretions. Naturwissenschaften 89:308-310.
Meinwald YC, Meinwald J, Eisner T. 1966. 1,2-Dialkyl-4(3H)-Quinazolinones in the defensive secretion of a millipede (Glomeris marginata). Science 154:390-391.
Pasteels JM, Theuring C, Witte L, Hartmann T. 2003. Sequestration and metabolism of protoxic pyrrolizidine alkaloids by larvae of the leaf beetle Platyphora boucardi and their transfer via pupae into defensive secretions of adults. J Chem Ecol 29(2): 337-355.
Radford AJ. 1975. Millipede burns in man. Trop Geogr Med 27:279-287.
Santori RT. 1998. Discrimination of millipedes by the opossum Didelphis albiventris (Marsupialia, Didelphidae). J Adv Zool 19(2):118-119.
Valderrama X, Robinson JG, Attygalle AB, Eisner T. 2000. Seasonal anointment with millipedes in a wild primate: a chemical defense against insects?. J Chem Ecol 26(12):2781-2790.
Williams LAD, Singh PDA, Caleb-Williams LS. 1997. Biology and biological action of the defensive secretion from a Jamaican millipede. Naturwissenschaften 84:143-144.
Wood WF, Hanke FJ, Kubo I, Carroll JA, Crews P. 2000. Buzonamine, a new alkaloid from the defensive secretion of the millipede, Buzonium crassipes. Biochem Syst Ecol 28:305-312.
Woodring JP, Blum MS. 1965. The anatomy, physiology and comparative aspects of the repugnatorial glands of Orthocricus arboreus (Diplopoda: Spirobolida). J Morphol 116:99-108.

